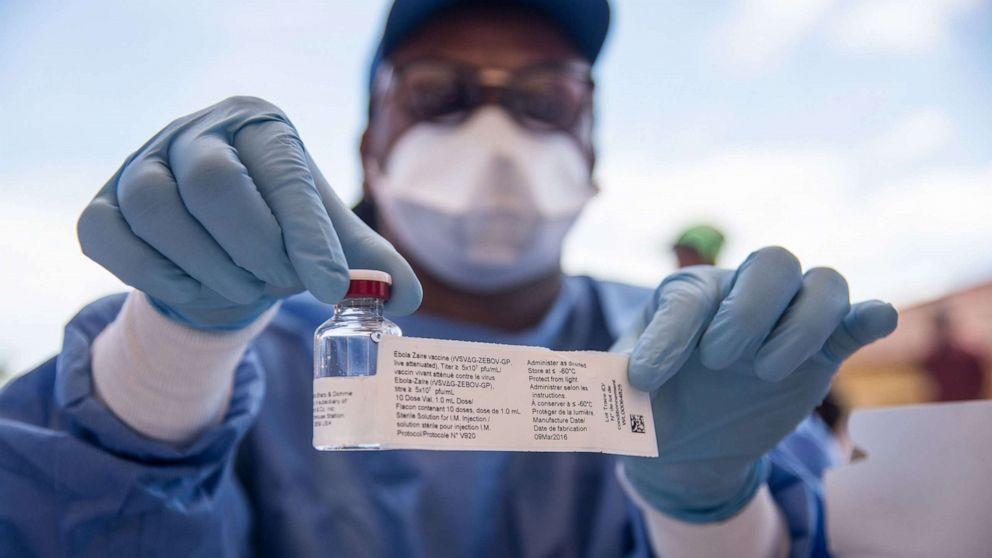
As global concerns mount over the threat of a new Ebola epidemic, the World Health Organisation (WHO) said it has commenced clinical trial against the Sudan ebolavirus—one of the six species of the Ebolavirus genus.
In a statement issued by its Regional Director for Africa, Dr. Matshidiso Moeti, WHO said the first doses of candidate vaccines against the Sudan ebolavirus were expected to arrive in Uganda in the coming days,
Moeti, who addressed a press conference in Uganda accompanied by the Incident Commander, Ebola outbreak, Ministry of Health, Uganda.Lt. Col. Henry Kyobe Bossa, said WHO was boosting efforts to support the government-led response against the outbreak which has now affected nine districts, including three complex urban environments.
The statement noted that WHO’s committee of external experts had evaluated three candidate vaccines and agreed that they all be deployed to Uganda for a clinical trial against the Sudan ebolavirus—one of the six species of the Ebolavirus genus.
It explained that unlike the Zaire ebolavirus which had sparked most of the recent outbreaks, there are no approved vaccines or therapeutics for the Sudan ebolavirus.
“The aim of the randomised trial is to evaluate potentially efficacious candidate vaccines, and to possibly contribute to ending the ongoing outbreak and protect populations at risk in the future.
“The trial is the result of a collaborative effort, coordinated by WHO with developers, academic institutions, countries’ sponsoring the production of the vaccine doses, regulatory authorities, other experts and the government of Uganda,” it said.
According to WHO, supplies of one of the three candidate vaccines were expected to arrive Uganda next week and the other two soon after.
It said trial protocol had been conditionally approved by WHO and Uganda and the final approvals are expected soon.
It added that import permits for the vaccines were expected to be issued by the National Regulatory Authority soon.
While the trial start date was not certain yet, WHO said it was working with the Ministry of Health and Makerere University, which is leading the trial to make sure everything was ready and the trial would begin once one vaccine has arrived and all the trial preparations are in place.
“The two other candidates will be added, as they become available. The start of vaccine trials will mark a pivotal moment towards the development of an effective tool against the virus behind the current Ebola outbreak in Uganda,” Moeti said.
She expressed hope that the vaccines would be effective in stopping Ebola spread in the same way it helped to check previous outbreaks, Moeti however said it would take time to get trial results, adding that for now the outbreak can be controlled without vaccines.
Uganda declared an outbreak of Sudan ebolavirus on 20 September. As of 14 November 2022, there had been 141 confirmed and 22 probable cases (total of 163 cases) and 55 confirmed and 22 probable deaths (77 total deaths) reported.
Nineteen health workers have been infected with the virus and seven have died.
On 11 November, the eastern Jinja district, which hosts Jinja city, became the third urban area—after the capital of Kampala and Masaka city—to detect the virus. Jinja, located on the shores of Lake Victoria, is home to some 300 000 people. While Jinja is now impacted by Ebola, the outbreak is slowing down in six districts, with two dropping from the follow-up list as they have reported no cases in over 42 days.
Source: thisdaylive